Osteopontin and Endometriosis
Oct 20, 2020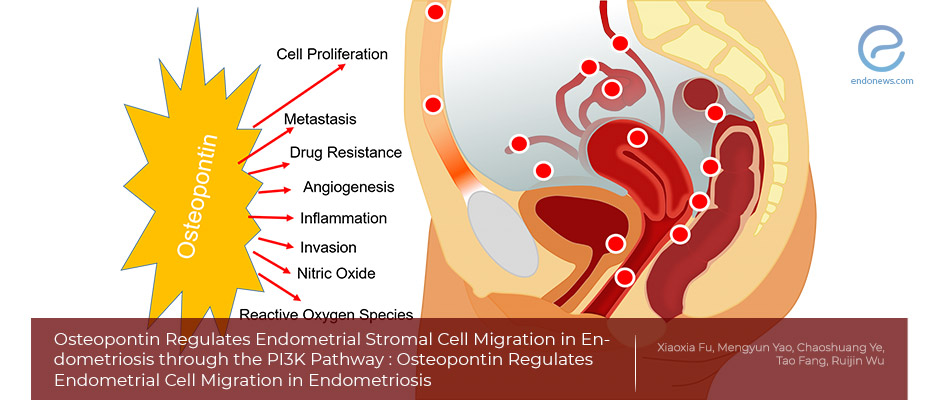
Osteopontin Regulates Endometrial Stromal Cell Migration in Endometriosis
Key Points
Highlight:
- Dr. Wu group demonstrated that osteopontin affects the migration of endometrial stromal cells through the PI3K signaling pathway.
Background:
- Osteopontin is one of the important molecules in cancer progression and metastasis, overexpressed in some cancers including endometrial cancer, and implicated in angiogenesis, cell adhesion, migration, and anti-apoptosis.
- Osteopontin also mediates the progression of ovarian cancer through activation of the PI3K/Akt pathway.
- Recently, it was shown that osteopontin is 130-fold higher in ectopic endometria compared to the eutopic endometrium, suggesting a critical role in endometriosis.
- However, the roles for osteopontin, PI3K signaling pathway still remain unexplored in the pathogenesis of endometriosis.
Key points:
- The level of Osteopontin is significantly higher in the sera of patients with endometriosis compared to the controls.
- When mRNA of osteopontin and uPA are concerned, the levels are higher in ectopic stromal cells compared to the eutopic endometrial stromal cells.
- The migration ability of ectopic endometrial stromal cells was significantly attenuated after osteopontin downregulation, while its knockdown showed no influence on cellular proliferation.
- The migration ability of ectopic endometrial stromal cells was significantly decreased by the PI3K pathway inhibitor and increased by the activator.
Conclusions:
- Osteopontin is upregulated in endometrial stromal cells from participants with endometriosis.
- Osteopontin regulates cellular migration of endometrial stromal cells by modulating uPA expression through the PI3K signaling pathway.
Lay Summary
Although characterized as a benign disease, some properties of endometriosis mimic malignant tissues such as proliferation, cell invasion, and metastasis. Osteopontin, also called secreted phosphoprotein 1, is one of the important molecular targets in cancer progression and metastasis. Osteopontin has been found in a variety of tissues including bone matrix, secretory glands, and some tumor tissues, and also secreted by activated immune cells.
A previous study illustrates that Osteopontin is overexpressed in a wide range of malignant tumors including endometrial carcinoma, and is implicated in anti-apoptosis, angiogenesis, cell adhesion, and migration. It was reported that Osteopontin mediates the progression of ovarian cancer through the activation of the PI3K/Akt pathway.
Recently, Osteopontin and endometriosis relation was also reported. Osteopontin levels were 130-fold higher in ectopic endometrial lesions compared to the eutopic samples, suggesting a critical role in endometriosis. However, the roles for Osteopontin, PI3K signaling pathway, and urokinase plasminogen activator still remain largely unexplored in the pathogenesis of endometriosis.
In this paper, Dr. Wu group from China, investigates the Osteopontin effect on the migration ability of endometrial stromal cells. This paper was recently published in the journal “Reproductive Sciences”.
The level of Osteopontin is significantly higher in the sera of endometriosis patients compared to the controls. Comparing to eutopic and ectopic endometrial stromal cells, ectopic cells have a higher mRNA level of Osteopontin and urokinase plasminogen activator. The migration ability of ectopic endometrial stromal cells was significantly attenuated after Osteopontin downregulation, whileOsteopontin knockdown showed no influence on cellular proliferation. Furthermore, the migration ability of ectopic endometrial stromal cells was significantly decreased by the PI3K pathway inhibitor.
In conclusion, the group successfully demonstrated that Osteopontin was upregulated in ectopic stromal cells from participants with endometriosis, demonstrating that Osateopontin regulates cellular migration of ectopic endometrial stromal cells by modulating urokinase plasminogen expression through the PI3K signaling pathway.
Research Source: https://pubmed.ncbi.nlm.nih.gov/32909189/
Osteopontin endometriosis cell migration PI3K uPA

