Cell-Aging-Related Pathways Linked to Endometriosis Pathogenesis
Nov 6, 2025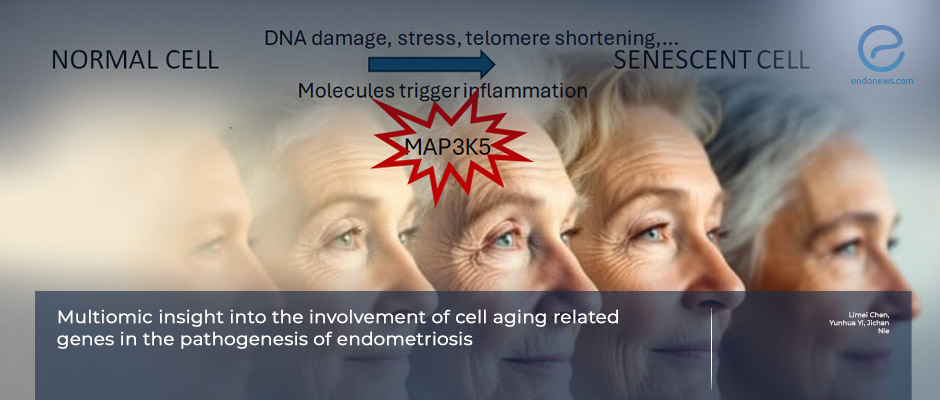
Multiomics Uncover Genetic and Epigenetic Bridges Between Cellular Senescence and Endometriosis
Key Points
Highlights:
- A multiomic analysis integrating transcriptomic, genetic, and epigenetic data identified cell-aging–related pathways as contributors to the development of endometriosis, pinpointing MAP3K5, an aging- and stress-response–related kinase gene.
Importance:
- While endometriosis has been extensively associated with inflammation and hormonal dysregulation, its connection with cellular aging and senescence pathways remains underexplored.
- This study provides genomic and epigenetic evidence supporting the hypothesis that aging-related mechanisms may underlie susceptibility and disease progression, opening new therapeutic perspectives.
What's Done Here:
- This is a multiomic integrative analysis combining transcriptomic data from endometriosis and control endometrial tissues.
- Genome-wide association data (GWAS) to identify causal gene–disease relationships and epigenetic (methylation) profiles to explore regulatory mechanisms were performed.
- Mendelian randomization and network analyses were used for investigating aging-related genes across these datasets to determine their causal impact on endometriosis risk.
Key Results:
- MAP3K5 emerged as a causal gene connecting aging-related molecular changes with endometriosis risk.
- Methylation and expression profiles supported MAP3K5 dysregulation in affected endometriotic tissues.
- The gene network analysis highlighted oxidative stress, apoptosis, and inflammatory signaling pathways as overlapping biological links between aging and endometriosis.
- These multiomic correlations strengthen the concept of endometriosis as a systemic and age-influenced disorder, not solely a localized pelvic disease.
Strength and Limitations:
- Strengths are integration of genomic, transcriptomic, and epigenetic data, providing a comprehensive systems-level insight.
- Limitations are that the analysis relies on publicly available datasets and requires experimental validation in primary endometrial or stromal cell models to confirm the proposed biological mechanisms.
From the Editor-in-Chief – EndoNews
"This study expands the scientific narrative of endometriosis beyond hormonal imbalance and inflammation toward the broader biological landscape of cellular aging. By integrating genomic, transcriptomic, and epigenetic layers, the authors bring forward a new mechanistic dimension—suggesting that premature activation of aging pathways may underlie the disease’s persistence, recurrence, and resistance to therapy.
The identification of MAP3K5 as a convergent hub between stress signaling, apoptosis, and inflammation highlights a possible bridge between molecular senescence and chronic pelvic disease. This finding invites us to reconsider endometriosis not only as a reproductive disorder but as a systemic condition shaped by altered cellular resilience and energy metabolism.
Although based on in-silico data, the work demonstrates how multiomic approaches can reveal patterns invisible to traditional analyses. It points toward a future in which targeting senescence-related signaling may complement hormonal or surgical strategies, broadening therapeutic horizons for women affected by this complex disease."
Lay Summary
The biology of endometriosis continues to challenge conventional understanding. While inflammation, hormonal imbalance, and immune dysfunction have long been recognized, this new study explores an entirely different dimension—cellular aging.
Researchers from Shanghai Obstetrics and Gynecology Hospital, used a multiomic approach that merged genomic, transcriptomic, and epigenetic data to uncover how aging-related molecular processes might contribute to the disease.
Published in Scientific Reports, the analysis drew on extensive public datasets from women with and without endometriosis to identify overlapping molecular patterns between senescence, oxidative stress, and inflammation. Using advanced statistical and network methods, the team identified MAP3K5, a kinase involved in stress signaling and apoptosis, as a central molecular node linking aging pathways with endometriosis risk.
This integrative strategy revealed that the same cellular stress responses driving tissue repair and fibrosis during normal aging may also perpetuate chronic inflammation and lesion persistence in endometriosis. Genes regulating mitochondrial energy balance, reactive oxygen species, and programmed cell death were found to be tightly interconnected, forming a biological bridge between aging biology and reproductive disease.
The findings open a new conceptual avenue—endometriosis may represent, at least in part, a disorder of accelerated cellular aging and impaired stress recovery.
This perspective not only redefines the disease’s molecular foundation but also suggests that targeting aging-related signaling pathways could become a novel therapeutic direction.
Approaches that modulate cellular senescence, oxidative balance, or mitochondrial health may one day complement hormonal or surgical treatments, offering a more durable solution for women suffering from this complex condition.
Research Source: https://pubmed.ncbi.nlm.nih.gov/40269081/
Endometriosis Multiomics aging Senescence MAP3K5 Oxidative stress Epigenetic Gene expression Mendelian randomization Inflammation Fibrosis

