Analogous pathways of peritoneal tumor wound healing and endometriosis.
Jun 24, 2019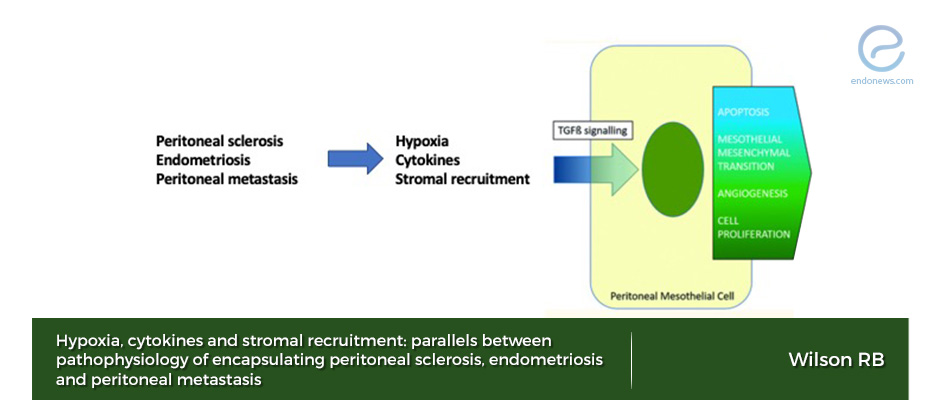
Chronic inflammation and endometriosis peritoneal lesions have similar features.
Key Points
Highlights:
- Tissue hypoxia, degraded cytokines, and stromal recruitment, all are the same steps of pathophysiologic pathways of encapsulating peritoneal sclerosis, endometriosis, and peritoneal metastasis.
Importance:
- Pathophysiology of wound healing of peritoneal surfaces due to endometriosis is very similar to other chronic inflammatory diseases which affect peritoneum.
What's done here:
- The author, an upper gastrointestinal surgeon, reviewed the pathophysiological mechanisms of peritoneal reaction after peritoneal injuries such as tumor metastasis, endometriosis, intraperitoneal chemotherapy, peritoneal dialysis.
Backgrounds:
- Wounds and tumors are both hypoxic and when cells experience a shortage of oxygen, they produce HIF (hypoxia-inducible factors). HIF induces angiogenesis to deliver oxygen and nutrition for healing the tissue.
- Both HIF-1α and HIF-β activate VEGF (vascular endothelial growth factor) in order to bind its receptor and stimulates neoangiogenesis. VEGF has multiple independent pathways which contribute to chronic inflammation, organ fibrosis, and carcinogenesis.
- If the initial injury is severe or repetitive, the wound becomes chronic and HIF, VEGF, TGF (transforming growth factor) families in the inflammatory process continue cellular proliferation, stromal recruitment, and angiogenesis.
- TGF-ß superfamily includes at least 40 functionally and structurally related proteins such as activins, inhibins, anti-Mullerian hormone, growth differentiation factors (GDFs). Persistently high expression of TGF-1ß or stimulation by inflammatory cytokines induce peritoneal mesothelial-mesenchymal transmission, adhesion formation, and fibrosis.
- Platelets contain 40-100 times TGF-1ß levels than other non-neoplastic human cells. Activated platelets release TGF-1ß, VEGF, PDGF, fibroblast growth factor, EGF, Insulin and some other growth factors from their cytoplasms.
Lay Summary
Endometriosis, diagnosed around 10-15% of women population in their reproductive period, is a chronically progressive inflammatory disease to cause peritoneal lesions while spreading into the abdominal cavity.
The pathophysiology of the adhesions between the peritoneum and pelvic organs can be explained by the similar pathways of some other clinical conditions such as peritoneal metastasis and injuries, which cause hypoxia, cytokines discharge, fibrosis, and sclerosis of peritoneal tissues.
Dr. Wilson, from the Upper Gastrointestinal Surgery Department of Liverpool Hospital, Australia, penned this article in the journal named "Pleura and Peritoneum" to examine and explain quite similar underlying pathophysiology of encapsulating peritoneal sclerosis, endometriosis, and peritoneal metastasis through the wound healing mechanisms which involve the peritoneal response to various kind of injuries that include mesothelial-mesenchymal transition and epithelial-mesenchymal transition.
All the above diseases are conditions associated with fibrosis of peritoneum and include hypoxia, damage signals, inflammatory cell migration, persistent secretion of cytokines, stromal growth factors, and chemokines; similar events to those lesions arising in endometriosis involving peritoneum.
Endometrium itself has stem cell-like renewal ability and this feature is under the influence of 17ß-Estradiol, resulting in cell proliferation in the proliferative phase of the menstrual cycle.
In the case of endometriosis, increased 17ß-Estradiol drives release of some cytokines like VEGF, HGF, IL-6, TNF-α from peritoneal macrophages. Markers of epithelial-mesenchymal transition, such as SLUG, SNAIL, TWIST1 are highly expressed in ectopic endometrium compared to eutopic endometrium. Increased expressions of MYC, Cyclin D1, and Ki67 genes also reflect higher level of proliferation and loss of cell cycle regulation in endometriosis.
The above findings indicate that the growth of endometrial-like lesions in peritoneal surfaces starts from basal membrane invasion through epithelial-mesenchymal transition. TGF- ß1 induced transformation and stromal recruitment paths are also similar in endometriosis and peritoneal metastasis.
The author has the same point of view with the 1986 postulation of Harold Dvorak and concluded that the dysregulated mechanisms of wound healing in encapsulating peritoneal sclerosis, endometriosis, and peritoneal metastasis are causing similar pathological pathways resulting in fibrosis in the peritoneum.
Research Source: https://www.ncbi.nlm.nih.gov/pubmed/30911653
hypoxia cytokines stromal recruitment peritoneal sclerosis CAPD DAMPs EPS HIF TGB-β1 VEGF peritoneal mesothelial cells hyaluronan peritoneal metastasis endometriosis.

