Unexpected "cancer-related" mutations in "non-cancerous" endometriosis: Do they predict aggressiveness?
May 28, 2017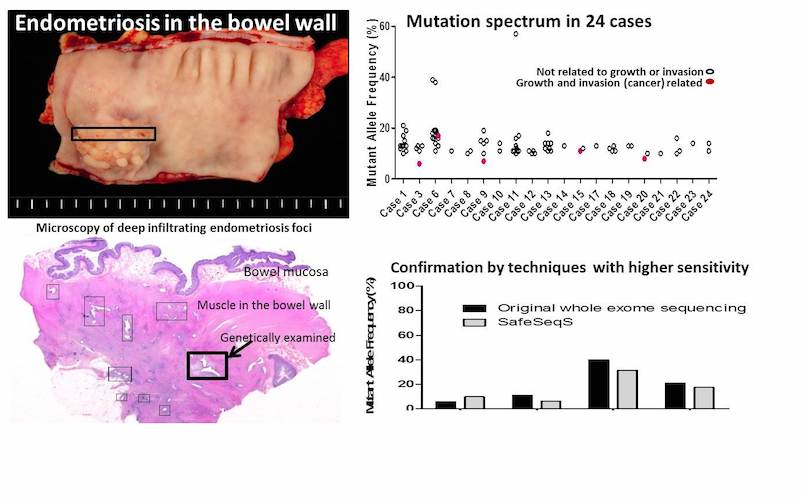
A collaborative study supported by Endometriosis Foundation of America, have identified the molecular alterations of deep infiltrating endometriosis trough a wide protein coding genetic-alphabet order study. Molecular alterations, specifically cancer-related gene mutations, have been identified.
Key Points
Highlights:
- Endometriosis is a well-known benign inflammatory disease.
- Some lesions, however, carry cancer-like features including local invasiveness and widespread dissemination.
- Molecular alterations, specifically cancer-related gene mutations, have been identified in a type of (so-called “Deep Infiltrating”) Endometriosis (DIE) which virtually carries a very low risk of malignant transformation.
Importance:
- This peculiar form of endometriosis (DIE) with invasiveness features and responsible of pain, infertility and specific site symptoms appear to carry non-random chromosomal alterations.
- This information will help us to show the way how to improve the understanding of endometriosis pathogenesis and classification.
- Mutational profile of the endometriotic tissues exploited in the future in order to stratify the disease and to identify those with more aggressive forms and even to provide personal treatment with target agents.
What’s done here?
- Exomes are the part of the genome responsible for coding protein synthesis. Here a wide genetic alphabet study of deep-infiltrating endometriosis samples was performed with further validation through highly sensitive genetic new methods.
- Past studies were conducted by lower-resolution genomic methods and have been mainly focused in endometriosis-associated ovarian carcinomas.
Key results:
- After filtering out normal gene variations, abnormal DNA alterations were detected in the majority of the deep infiltrating endometriosis tissue samples (19 out of 24; 79%)
- Some of these DNA changes involve the genes that confer a selective growth advantage to the cell (promoting growth and invasion, such as ARID1A, PIK3CA, KRAS, and PPP2R1; and usually typically seen in cancers)
- It is important that those mutations alone are not sufficient to induce cancer transformation in deep infiltrating endometriosis.
Reference: Cancer-Associated Mutations in Endometriosis without Cancer. Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. N Engl J Med. 2017 May 11;376(19):1835-1848. doi: 10.1056/NEJMoa1614814. PMID: 28489996
Lay Summary
A study supported by Endometriosis Foundation of America, reported by scientists from Johns Hopkins Medicine (Baltimore, USA) and the University of British Columbia (Vancouver, Canada) have identified the molecular alterations of deep infiltrating endometriosis trough a wide exome sequencing (protein-coding genetic alphabet order) study and then validation through other highly sensitive digital genomics.
Three main clinical forms of endometriosis are superficial peritoneal endometriosis, ovarian endometriosis, and deep infiltrating endometriosis (DIE). Even if endometriosis itself is a benign disease, shares some features with malignancy, in especially DIE which is characterized by invasion and dissemination of pelvic structures causing severe pain, infertility and site-specific symptoms. There is desperate need to better understand the creation and generation of these processes and the molecular alterations involved in the development of this form of endometriosis.
They analysis included DIE lesions of 27 patients, collected from USA (New York, Departments of Obstetrics and Gynecology and Pathology at Lenox Hill Hospital–Northwell Health), Japan (Seirei Mikatahara Hospital in Hamamatsu) and Canada (BC Women’s Centre for Pelvic Pain and Endometriosis in Vancouver).
Interestingly they found the presence of mutations in the DIE lesions of the vast majority of the patients (19 of 24 patients; 79%) while they were not present in the normal tissue of the same patient. The type and number of mutations varied between patients and between distinct lesions in the same patient.
Surprisingly, in five of the patients, they identified mutations in cancer driver genes, such as ARID1A, PIK3CA, KRAS, and PPP2R1, genes involved in cell growth control, invasion and DNA damage repair. It is known that mutations in these genes favor the development of different types of cancers, including those arising from malignant transformation of ovarian endometriosis, for instance, Clear Cell Ovarian Carcinoma.
Curiously, however, even if deep infiltrating endometriosis and endometriosis-related ovarian carcinoma harbor similar mutations, malignant transformation of deep infiltrating endometriosis rarely occurs. More likely the presence of driver mutations alone is not sufficient to drive the malignant transformation of endometriosis; additional DNA damages are required for tumor development.
The identification of somatic mutations in deep infiltrating endometriosis raises many questions and suggests further studies. It will be intriguing to understand if those somatic mutations are present also in progenitors, for example in the eutopic endometrium of the patient, and in other forms of endometriosis. Most importantly, it should be investigated if variable patterns of somatic mutation correlate with severity, progression, and recurrence of the disease, in order to provide a “molecular signature” that identify patients with a bad outcome, requiring more intensive treatment approaches.
Lastly, the characterization of the molecular alterations could be exploited in the future for “personal disease treatment” with the use of agents that could block specific gene-related pathways involved in the development of deep-infiltrating endometriosis.
Research Source: Cancer-Associated Mutations in Endometriosis without Cancer https://www.ncbi.nlm.nih.gov/pubmed/28489996
mutation somatic passanger driver cancer-related deep infiltrating endometriosis Genes DNA damage cell growth cancer endometriosis-cancer

