Estrogen Receptors and Endometriosis: What do we know now?
Jul 2, 2020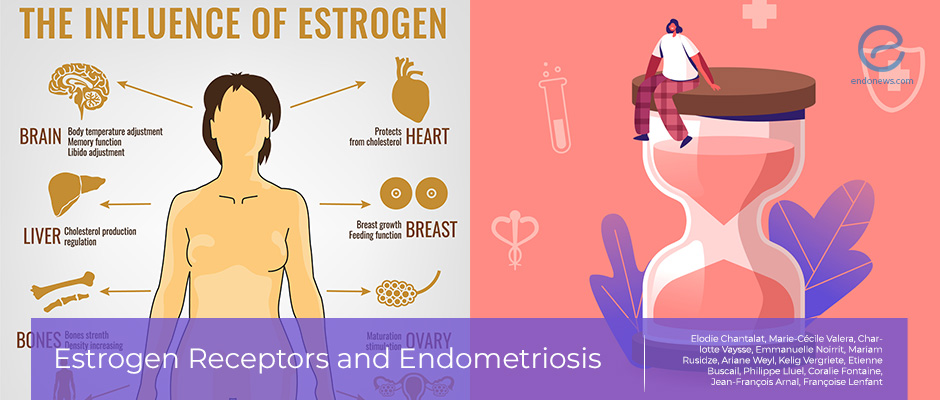
Estrogen, the complicated female sex hormone
Key Points
Highlight:
- Endometriosis is highly dependent on estrogen. Despite years of research, the current understanding of estrogen receptors (ER) in endometriosis pathogenesis still requires more investigations.
Importance:
- A greater understanding of the roles of ERs in endometriosis may lead to future targetable therapies which related directly to the endometriosis pathogenesis.
What has been done:
- This is a review article which provides an overview of the current understanding of the relationships between endometriosis and ERs.
- Recent advances in treatment strategies based on ERs modulation are summarized.
Results:
- Endometriosis is estrogen-dependent.
- High and dysregulated estrogen production is an important feature of endometriosis.
- The levels of ERs are altered between both normal healthy endometrium and all ectopic/eutopic endometrial lesions.
- Stromal cells in endometriotic tissues express remarkably higher ERβ and lower ERα levels as compared to the stromal cells from the endometrium of patients without endometriosis.
- There are a large number of studies regarding ERs in endometriosis; however, understanding of the roles of ERα and ERβ in the pathogenesis of endometriosis remains imperfect.
Lay Summary
It has been well known that endometriosis is closely associated with steroid hormone metabolism and pathways. Estradiol promotes the growth and progression of endometriotic tissue, together with the associated inflammation and pain-related symptoms.
Excess estradiol in endometriosis is mainly produced locally by the endometriotic tissue. This is because of the higher expression of critical enzymes for estrogen biosynthesis in endometriotic tissue, including aromatase (CYP19A1) and steroidogenic acute regulatory protein (StAR). Unlike in endometriosis, normal endometrium does not have the ability to synthesize estrogen as it lacks these enzymes. The accumulation of local estrogen acts to support the growth of endometriotic lesions by binding and activating estrogen receptors (ERs) in the cells membrane.
There are two subtypes of ERs, namely, ERα and ERβ, which are encoded by the genes estrogen receptor 1 (ESR1) and 2 (ESR2), respectively. These receptors can enter the cell nucleus and regulate the activity of different genes, thereby regulating biological functions in many ways.
This review article by Chantalat and Valera et al. from Université de Toulouse, France aims to provide the current understanding of the mechanisms of action of ERs in the progression of endometriosis and discusses new therapeutic strategies developed based on knowledge regarding estrogen. The article was published recently in the "International Journal of Molecular Sciences".
Many studies performed in mouse models and in cells isolated from women with endometriomas have suggested the contribution of each ER subtype in the initiation and progression of endometriosis. Together, these data show that ERα and ERβ act in several ways to promote endometrial cell proliferation and tissue-invasion at ectopic lesions. While both ER subtypes are involved, recent data seem to point to the central role for ERβ. Specifically, the excess estradiol in endometriosis support ERβ signaling, which then leads to endometriotic tissue survival and inflammation. ERβ may also cause damage through non-estradiol-related mechanisms.
Due to the significant role of estrogen in endometriosis pathology, there are now a number of drug examining other therapeutic strategies that exploit estrogen and ERs, in addition to the currently available treatments for endometriosis, which inhibit ovarian estradiol production by using contraceptive steroids, GnRH agonists, progestins, or aromatase inhibitors. These new treatments that are in development include ERβ-selective compounds that act as estradiol antagonists and molecules that target tumor necrosis factor (TNF) action e.g., recombinant TNF receptor type-1 because of the established link between overexpression of ERβ and inhibition of TNFα-mediated apoptosis.
Furthermore, estrogen induces cyclooxygenase 2 (COX2) function to mediate inflammation via ERβ. Efforts to disrupt COX2 functions include selective and nonselective inhibitors, which were shown to reduce pelvic pain in endometriosis. Other ER ligands are also under investigation, such as selective ERβ ligand (chloroindazole) and the ERα antagonist (oxabicycloheptene sulfonate). Selective estrogen receptor modulators (SERMs) are other synthetic molecules that bind to ERs e.g., Raloxifene. Some of these new ligands showed anti-estrogenic and anti-inflammatory activities in endometriotic cells. However, it must be noted that studies performed were preclinical and their efficacy needs much further clinical testing if warranted.
In summary, decades of studies have equivocally shown that estrogen and ERs are important in endometriosis pathogenesis. Nonetheless, there are still many open questions relating to ERs specific mechanism of actions in endometriosis. For example, while it is well known that endometriosis is a chronic inflammatory disease, the roles of ERαand ERβ in mediating the inflammatory response are unclear. Therefore, the full understanding of ERα and ERβ in endometriosis is still incomplete.
It is hoped that a better understanding of ER mechanisms will lead to superior therapies that target both the endocrine and inflammatory pathways.
Research Source: https://pubmed.ncbi.nlm.nih.gov/32316608/
treatment hormone pathogenesis

