Mast Cell–Mediated Epithelial–Mesenchymal Transition in Endometriosis
Oct 24, 2025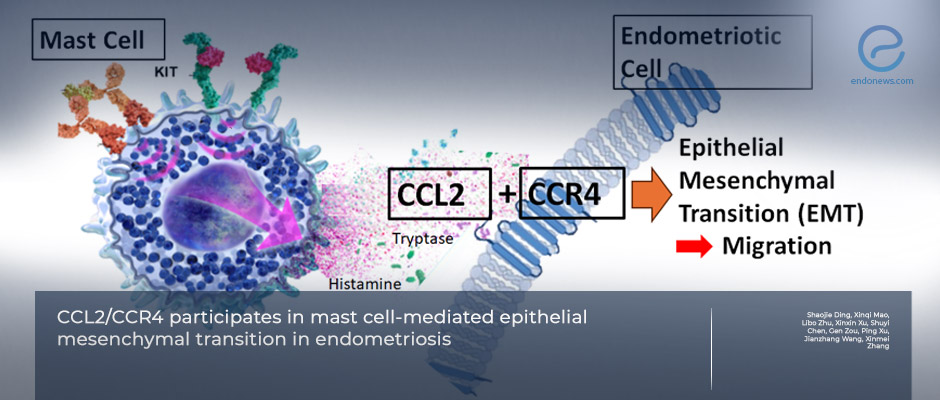
Mast Cells Promote Endometriosis Progression Through CCL2/CCR4-Driven EMT
Key Points
Highlights:
- Mast cells contribute directly to the pathogenesis of endometriosis through the epithelial–mesenchymal transition (EMT) process.
- The MRGPRX2/CCL2/CCR4 signaling pathway mediates mast cell–induced EMT in endometrial epithelial cells.
- Inhibition of this pathway effectively suppresses EMT and endometriosis progression in vitro and in vivo.
Importance:
- This study establishes a mechanistic link between inflammation and cellular remodeling in endometriosis.
- Targeting mast cell activation or CCL2/CCR4 signaling may offer a novel therapeutic approach to control disease progression and recurrence.
What's Done Here?
- Researchers analyzed human ovarian endometriosis samples (n = 18) and controls (n = 13), isolating endometrial epithelial cells for co-culture with a human mast cell line (HMC-1.1).
- Mast cell activation and EMT marker expression were quantified using qRT-PCR, Western blot, and immunofluorescence.
- A wild-type vs. mast-cell-deficient mouse model of endometriosis was used to test in-vivo effects.
- Specific inhibitors of MRGPRX2, CCR4, and CCR2 were applied to verify the mechanistic pathway.
Key Results:
- Activated mast cells were strongly correlated with EMT markers in human endometriotic lesions (↑ CDH2, ↓ CDH1).
- Mast cells promoted migration and EMT of endometrial epithelial cells via MRGPRX2/CCL2/CCR4 signaling.
- Pharmacologic inhibition of this pathway reduced EMT induction in vitro.
- In vivo, mast-cell deficiency suppressed endometriosis lesion growth and EMT marker expression.
- Findings collectively indicate that mast-cell-derived CCL2 acts through CCR4 to drive EMT and lesion development.
Limitations:
-
Small cohort (n = 31) limited to ovarian endometriosis may not represent other phenotypes; protease inhibitors were not added during fluid collection, which may have affected cytokine quantification; further studies are needed to validate therapeutic applicability and cross-species pathway consistency.
From the Editor-in-Chief – EndoNews
"This paper convincingly connects mast-cell activation to EMT-driven lesion biology in endometriosis through the MRGPRX2 → CCL2 → CCR4 axis. The multi-tier design—human lesions, mechanistic co-culture, and a mast-cell-deficient mouse model—adds weight to causality rather than mere association. Clinically, the work reframes endometriosis as a disorder of immune–epithelial crosstalk, not just ectopic endometrium plus hormones.
Two translational paths emerge: (1) therapeutics—testing MRGPRX2 antagonists or CCR4 inhibitors as adjuncts to hormonal or surgical care; and (2) biomarkers—evaluating peritoneal/serum CCL2 and mast-cell signatures to identify patients likely to benefit from anti-inflammatory, anti-EMT strategies. Caution is warranted: phenotypic diversity (ovarian vs. peritoneal vs. deep infiltrating disease) and safety profiles of pathway inhibitors must be addressed prospectively. Nonetheless, by mechanistically tying inflammation to invasiveness, this study charts a credible route toward immune-targeted, relapse-minded therapy in endometriosis."
Lay Summary
A new study led by Dr.Zhang in Reproductive BioMedicine Online reports that mast cells—immune cells best known for triggering allergy symptoms—may also drive the progression of endometriosis by pushing uterine cells into a more invasive state called epithelial–mesenchymal transition (EMT). EMT is a cellular program in which orderly, “brick-like” epithelial cells loosen their bonds, become more spindle-shaped, and move more easily—traits that can help endometrial cells implant and spread outside the uterus.
The researchers combined three lines of evidence. First, in human tissue, endometriotic lesions contained more activated mast cells, and the number of these cells correlated with EMT marker patterns (higher N-cadherin, lower E-cadherin). Second, in cell culture, normal endometrial epithelial cells were co-cultured with a human mast-cell line. Mast-cell activation increased cell migration and EMT, and this effect depended on a signaling route that starts at a mast-cell surface receptor (MRGPRX2) and releases the chemokine CCL2, which then acts on CCR4 receptors to change epithelial behavior. When the team blocked MRGPRX2 or CCR4 pharmacologically, EMT signals and cell movement dropped. Third, in a mouse model engineered to have fewer or inactive mast cells, endometriosis lesions were smaller and less EMT-like, suggesting mast-cell activity is important for disease growth in vivo.
Most current therapies for endometriosis rely on hormones or surgery, and recurrence is common.This work points to a new therapeutic angle—targeting mast-cell activation or the CCL2/CCR4 pathway—that could complement existing treatments and potentially reduce lesion progression or pain. The authors note limitations, including the relatively small human cohort and focus on ovarian endometriosis; larger, multi-phenotype studies will be needed. Still, the convergence of human, in-vitro, and animal data provides a persuasive mechanistic link between inflammation and tissue remodeling in endometriosis.

