Autophagy dysregulation reshapes our understanding of endometriosis biology
Jan 9, 2026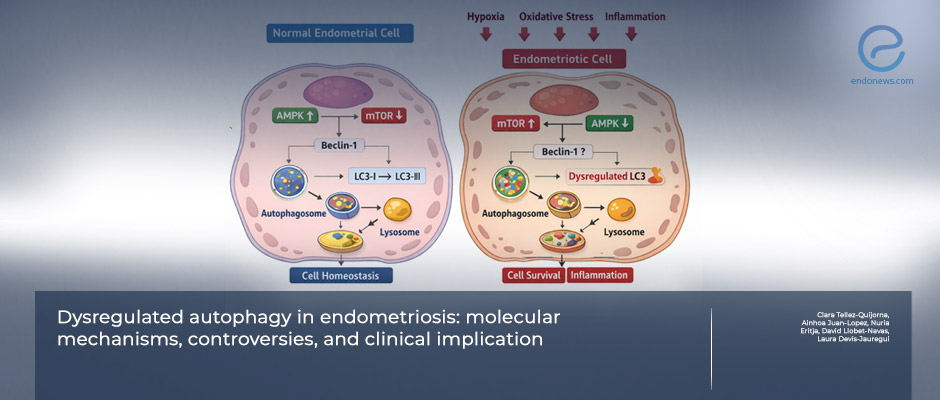
When cellular recycling fails: autophagy dysfunction in endometriosis
Key Points
Highlights:
- Autophagy plays a complex and context-dependent role in the development and persistence of endometriosis.
- Evidence suggests that both excessive and insufficient autophagic activity may contribute to lesion survival, inflammation, and treatment resistance.
- Crosstalk between autophagy, estrogen signaling, oxidative stress, immune dysfunction, and metabolic adaptation is central to disease biology.
Importance:
- Clarifying how autophagy is regulated in endometriosis is essential to determine whether it represents a pathogenic mechanism, an adaptive response, or a viable therapeutic target.
What's Done Here?
- The authors critically review experimental and clinical studies examining autophagy in endometriosis.
- Data from human tissues, animal models, and in vitro systems are integrated to evaluate molecular pathways, regulatory signals, and disease-stage specificity.
- Key autophagy regulators—including mTOR, AMPK, Beclin-1, LC3, and lysosomal pathways—are discussed in relation to lesion biology.
Outline:
- Endometriotic tissues frequently exhibit altered expression of autophagy-related markers compared with eutopic endometrium.
- Autophagy appears to support cell survival under hypoxia, oxidative stress, and inflammatory conditions characteristic of ectopic lesions.
- Estrogen signaling and immune-mediated pathways modulate autophagic flux, potentially contributing to lesion persistence and progesterone resistance.
- Preclinical studies suggest that modulating autophagy may influence lesion growth, but findings remain heterogeneous and context-dependent.
Limitations:
- Available evidence is largely derived from preclinical models with variable methodologies and inconsistent markers of autophagic flux.
- Human data are limited, and causal relationships between autophagy and disease progression remain unproven.
- Clinical trials directly targeting autophagy in endometriosis are currently lacking.
- Neuropathic-like pain was not associated with endometriosis stage, surgical complexity, or anxiety and depression scores.
From the Editor-in-Chief – EndoNews
This review provides a timely and necessary recalibration of how autophagy is interpreted in endometriosis research. Rather than presenting autophagy as a unidirectional pathogenic mechanism or an obvious therapeutic target, the authors emphasize its context-dependent and often contradictory behavior across experimental systems. This balanced perspective is particularly valuable in a field where mechanistic enthusiasm has frequently outpaced clinical validation.
A central contribution of the review is its integration of autophagy into the broader biological landscape of endometriosis. Autophagic pathways are shown to intersect with estrogen signaling, inflammatory and immune responses, oxidative stress, and metabolic adaptation—processes already known to shape lesion survival. In this framework, altered autophagy appears less as an isolated abnormality and more as part of a cellular survival program activated in response to hostile ectopic environments.
Equally important is the review’s critical appraisal of existing evidence. Variability in disease models, methodological approaches, and markers used to assess autophagic flux limits comparability across studies and complicates causal interpretation. The authors appropriately caution against equating changes in autophagy-related markers with functional relevance, underscoring a common pitfall in translational research.
From a clinical standpoint, the review tempers expectations. While preclinical data suggest that modulating autophagy can influence lesion behavior, the absence of robust human data and interventional trials precludes therapeutic conclusions. This restraint strengthens the review’s credibility and redirects attention toward defining when, where, and in whom autophagy is biologically meaningful.
Overall, this work advances the field by shifting the conversation from whether autophagy is involved in endometriosis to how its regulation integrates with disease heterogeneity. Future progress will depend on standardized methodologies, functional validation, and careful alignment of experimental models with clinically relevant phenotypes—steps essential before autophagy can be credibly translated into patient care.
Lay Summary
Autophagy is a normal cellular process that helps cells survive stress by breaking down and recycling damaged components. In recent years, this process has attracted growing attention in endometriosis research, as endometriotic lesions are exposed to challenging conditions such as inflammation, oxidative stress, low oxygen levels, and hormonal imbalance.
In this review, the authors examine current evidence on how autophagy behaves in endometriosis and whether it contributes to disease development or persistence. Studies from human tissue, animal models, and cell-based experiments suggest that autophagy is frequently altered in endometriotic lesions compared with normal endometrium. Rather than following a single pattern, autophagy appears to be dysregulated, with both increased and impaired activity reported depending on the biological context.
Importantly, autophagy may help endometriotic cells survive hostile environments, evade immune clearance, and adapt metabolically—features that support lesion persistence. Hormonal signaling, particularly estrogen dominance, as well as inflammatory and immune pathways, further influence autophagic responses in endometriosis.
However, the review also highlights major gaps in knowledge. Differences in experimental models, markers used to assess autophagy, and disease stages studied make it difficult to draw firm conclusions. At present, there is insufficient clinical evidence to support targeting autophagy as a treatment strategy.
Overall, the authors emphasize that autophagy should be viewed neither as a simple cause of endometriosis nor as a ready-made therapeutic target. Instead, a more nuanced understanding of when and how autophagy is altered may be essential for translating these findings into meaningful clinical advances.

